Answer:
The mass percent of acetic acid in the vinegar is 4,71% (m/m)
Step-by-step explanation:
Vinegar is an aqueous solution of acetic acid and other different chemicals that are flavorings. Vinegar typically contains 5% acetic acid by volume. That means 5mL of acetic acid per 100mL of solution.
To obtain the mass percent of acetic acid you must convert mL of acetic acid and solution to grams with densitiy.
The density of solution is 1,01 g/mL and the density of acetic acid is 1,05 g/mL. Thus, mass percent in vinegar is:
 ×
×
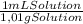 ×
×
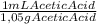 ×100 = 4,71% (m/m)
×100 = 4,71% (m/m)
I hope it helps!