Step-by-step explanation:
According to law of conservation of mass, total mass of reactants will be equal to the total mass of products.
For example,

Therefore, mass of reactant molecules will be as follows.
Mass of HCl is 36.46v g/mol
Mass of NaOH is 39.99 g/mol
Total mass of reactants is (36.46 + 39.99) g/mol = 76.45 g/mol
Mass of product molecules will be as follows.
Mass of NaCl is 58.44 g/mol
Mass of
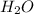 is 18.01 g/mol
is 18.01 g/mol
Total mass of products is (58.44 + 18.01) g/mol = 76.45 g/mol
Thus, we can conclude that any equation which represents total mass of reactants equal to the total mass of products is actually obeying the law of conservation of mass.