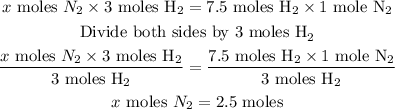Answer
2.5 moles of N₂ are needed to react with 7.5 moles of hydrogen gas
Step-by-step explanation
Given:
Equation: N₂ + 3H₂ → 2NH₃
Moles of H₂ = 7.5 moles
What to find:
The moles of nitrogen gas (N₂) needed to react with 7.5 moles of hydrogen gas
Step-by-step solution:
Let the mole of N₂ needed be x.
From the given balanced chemical equation:
3 moles of H₂ react with 1 mole of N₂
Therefore, 7. 5 moles of H₂ will react with x moles of N₂
Cross multiply