Answer:
The number of days is approximately 8.
Explanation:
Given : A medical scientist has a 15-gram sample of I-13, And would like to know it's half-life in days. he also knows that k=0.0856.
To find : The half-life, in days, of I-131 using the information at the left?
Solution :
The decay model is given by
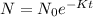
We have given that,
The substance's half-life is the time it takes for the substance to decay to half its original amount.
i.e.
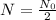
The value of k is k=0.0856.
Substitute the values in the formula,
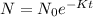
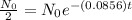
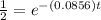
Taking natural log both side,
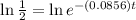
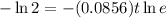
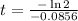
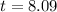
Therefore, The number of days is approximately 8.