staals half
1 gram left
0.3 grams left
half lives are normally written in years
it goes likt this
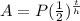
A=final amount
P=initila amount
t=time elapsed
h=time half life is (same units as time)
let's use days as the units of time
we are given
initial amount=1gram
final amount=0.3gram
time=4 days
so
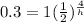
solve for h
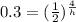
take the ln of both sides
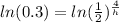
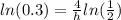
times both sides by h
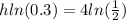
divide both sides by ln(0.3)
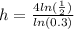
use calculator
h=2.30287
3 sig figs
h=2.303 days