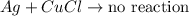Answer: A) No reaction would occur.
Explanation:
Single replacement reaction is a chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
Example:
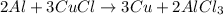
Thus When aluminium is treated with copper chloride ,aluminium being more reactive than copper displaces copper from its salt solution and thus produce aluminium chloride.
But when aluminum is replaced by silver, o reaction would occur as silver is less reactive than copper and hence would not be displace it from its salt solution.