Answer:
(d) is the correct option ''
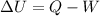 ''
''
Step-by-step explanation:
The first law of thermodynamics states the law of conservation of energy i. e. heat energy can neither be created nor be destroyed. The mathematical expression that show the first law of thermodynamics is as follows:
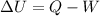
Where
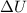 is the change in internal energy
is the change in internal energy
Q is the amount if heat added to the system
W is the work done by the system
Hence, the correct option is (d)