Answer:
Atomic mass of the element = 80.80 amu
Step-by-step explanation:
Given:
Mass of element = 4.00 g
No. of atoms =
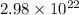
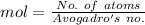
Avogadro's no. = tex]6.02\times 10^{23}[/tex]
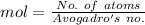
=
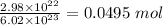
Formula for moles in the terms of mass and atomic mass
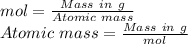
Atomic mass of the element =
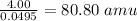
Atomic mass of the element = 80.80 amu