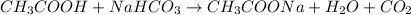Water breaks down into hydrogen and oxygen: It is a decomposition reaction as a single substance decomposes to give two products.
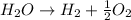
Leaves make starch using chlorophyll and carbon dioxide: Synthesis reaction: as the synthesis reaction involves two or more than two reactants which join together to result into a single main product along with the formation of simple by products.
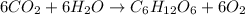
Food burns in oxygen gas and releases a lot of energy: Combustion: Combustion process involves the use of oxygen to give products along with release of energy.
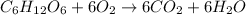
Adding vinegar (acid) to baking soda (alkali) gives a product that is neither acidic nor alkaline: Neutralization: acetic acid in vinegar reacts with soda (base) to give salt (neutral) and .