Answer: Option (c) is the correct answer.
Step-by-step explanation:
When calcium corbonate and hydrochloric acid react then the reaction will be as follows.
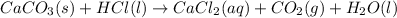
This reaction shows a chemical change as the calcium carbonate has transformed into calcium chloride, that is, the chemical composition of calcium carbonate has changed accompanied by the formation of carbon dioxide gas and water.
Thus, we can conclude that this reaction represents a chemical change because gas formation was observed, which indicated that the calcium carbonate was transformed into a different substance.