Answer:
If we consider a solution density of 1 g/ml, it is 0.9 g/100ml
Step-by-step explanation:
A solution 0.9% glucose can be expressed as follows:
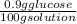
If the density of the solution (at a given temperature) is 1 g/ml, the solution concentration can be calculated as follows:
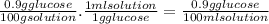
So, to calculate a solution concentration in g/ml from a %w/w concentration you have to only divide it into the solution density. Remember that solution density depends on the temperature.