Answer: The correct answer is
![k[NO_2][F_2]](https://img.qammunity.org/2018/formulas/chemistry/high-school/b8e9y8js5lldri9wb0wvrwyumaea107mr3.png)
Step-by-step explanation:
Rate law states that the rate of a reaction is directly proportional to the concentration of the reactants each raised to power a stoichiometric coefficient which is determined experimentally and is called as order.
![Rate=k[A]^x[B]^y](https://img.qammunity.org/2018/formulas/chemistry/high-school/uko51v6uamnvkf269vhkrfqfwvmb07en66.png)
where,
k = Rate constant
x = Order with respect to A
y = Order with respect to B
In a mechanism, it is determined from the slow step of the reaction.
The slow step of the mechanism is:
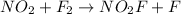
The rate of this reaction is given by the expression:
![rate=k[NO_2][F_2]](https://img.qammunity.org/2018/formulas/chemistry/high-school/8u90i888trkwq9r1g0m4g07qzzzlw76jh1.png)
Thus, the correct answer is
![k[NO_2][F_2]](https://img.qammunity.org/2018/formulas/chemistry/high-school/b8e9y8js5lldri9wb0wvrwyumaea107mr3.png)