Answer:
The half life of the substance is 6.0 days.
Explanation:
The initial equation for the initial mass and mass at time t is;
N =
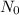
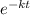
Where N is the mass at time t,
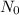 is the initial mass, k is the constant and t is the half life. After the half life i.e t,
is the initial mass, k is the constant and t is the half life. After the half life i.e t,
So that at a given time t,
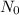 = 33 grams and N = 16.5 grams
= 33 grams and N = 16.5 grams
⇒ 16.5 = 33
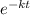
16.5 =
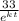
cross multiply, we have;
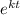 =
=
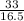
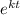 = 2
= 2
Find the natural logarithm of both sides,
ln
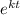 = ln2
= ln2
kt = ln2
⇒ t =
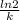
t =
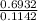
t = 6.07
The half life of the substance is 6.0 days.