Answer: The density of metal is 7.83 g/ml
Solution :
Given, Mass of metal = 105 g
First we have to calculate the volume of sand
Volume of metal = volume of water displaced= (83.3-69.9)=13.4 ml
Now we have to calculate the density of metal.
Formula used :
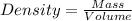
Now put all the given values in this formula, we get the density of metal.
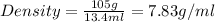
Therefore, the density of metal is 7.83 g/ml