Answer : Energy needed, Q = 0.3 cal
Explanation :
Given that,
The specific heat capacity of silver, c = 0.06 cal/g °C
Mass of the sample, m = 5 grams
It changes its temperature from 10°C to 11°C,
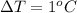
Energy need to flow is given by :
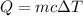
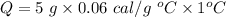
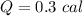
So, the energy needed to flow into this sample is 0.3 cal
Hence, this is the required solution.