Answer:
46.98 grams of cisplatin can be made from 65 grams of potassium tetrachloridoplatinate(II).
Step-by-step explanation:
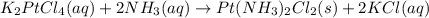
Mass of potassium tetrachloridoplatinate(II) = 65 g
Moles of potassium tetrachloridoplatinate(II) =
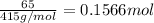
1 mol of potassium tetrachloridoplatinate(II) gives 1 mol of cisplatin.
Then 0.1566 moles of potassium tetrachloridoplatinate(II) will give:
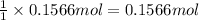
Mass of 0.1566 moles of cisplatin:
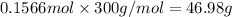
46.98 grams of cisplatin can be made from 65 grams of potassium tetrachloridoplatinate(II).