Answer: The correct answer is option (C).
Step-by-step explanation:
Sodium metal reacts with hydrochloric acid which produces hydrogen gas and sodium chloride.
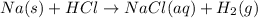
RHS = Products, LHS = Reactants
The reactants are sodium metal and hydrochloric acid.
The product are hydrogen gas and sodium chloride solution.
Hence,the correct answer is option (C).