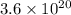 atoms of silicon weigh approximately 0.169 grams.
atoms of silicon weigh approximately 0.169 grams.
To calculate the weight of a given number of atoms, you can use the concept of molar mass and Avogadro's number.
1. Find the molar mass of silicon (Si). The molar mass is the mass of one mole of a substance. The atomic mass of silicon is approximately 28.09 g/mol.
2. Determine Avogadro's number, which is the number of atoms or molecules in one mole. Avogadro's number is
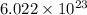 entities/mol.
entities/mol.
3. Calculate the weight (mass) using the formula:
![\[\text{Weight (g)} = \left(\frac{\text{Number of atoms}}{\text{Avogadro's number}}\right) * \text{Molar mass}\]](https://img.qammunity.org/2018/formulas/chemistry/high-school/x6v6a4xsjgo2g2819vt7qxp2o4sasswh25.png)
For silicon:
![\[\text{Weight (g)} = \left((3.6 * 10^(20))/(6.022 * 10^(23))\right) * 28.09 \, \text{g/mol}\]](https://img.qammunity.org/2018/formulas/chemistry/high-school/9u7p7jfswjxppjc9gdipnafxnmnzw4zyhk.png)
Now, let's calculate:
![\[\text{Weight (g)} \approx \left((3.6 * 10^(20))/(6.022 * 10^(23))\right) * 28.09 \, \text{g/mol} \approx 0.169 \, \text{g}\]](https://img.qammunity.org/2018/formulas/chemistry/high-school/6qkvqk9a75zsm4uvzm1um61q8jb0o8sm42.png)
Therefore,
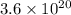 atoms of silicon weigh approximately 0.169 grams.
atoms of silicon weigh approximately 0.169 grams.