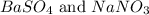Answer: The products formed are
Step-by-step explanation:
The reaction between barium nitrate and sodium sulfate is a type of double displacement reaction.
Double displacement reactions are defined as the reactions in which exchange of ions takes place. General equation for this type of reaction follows:
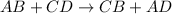
For the reaction of barium nitrate and sodium sulfate, the equation follows:
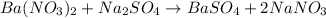
By Stoichiometry,
1 mole of barium nitrate reacts with 1 mole of sodium sulfate to produce 1 mole of barium sulfate and 2 moles of sodium nitrate.
Hence, the products formed are