Answer: The pressure of an ideal gas 0.278 atm.
Step-by-step explanation:
Volume of the ideal gas ,V = 35.5 L
Pressure of an ideal gas,P = 0.138 atm
Temperature of an ideal gas = 223 K
Number of moles of an ideal gas = 0.540 mol
Ideal gas equation is given as:
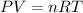
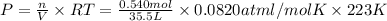
P = 0.278 atm
The pressure of an ideal gas 0.278 atm.