Promethium is the 61st element that you can find in the periodic table and the number 147 indicates the atomic mass.
Remember that the atomic number (in this case, 61) represents the number of protons, so there are 61 protons in Pm-147.
And to find the number of neutrons, we have to subtract the atomic number (61) from the atomic mass (147):
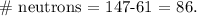
So, the final answer is that we have 61 protons and 86 neutrons of Pm-147.