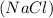Answer : The products form in the given reaction are, calcium carbonate,
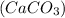 and sodium chloride,
and sodium chloride,
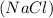
Explanation :
Double-displacement reaction : It is a reaction in which an aqueous ionic compounds exchange their ions (a positive cation and a negative anion) to form a new compounds.
In general,
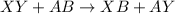
In this, X and A are the cations and Y and B are the anions.
The given balanced reaction is,
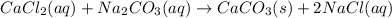
In the given reaction, calcium chloride exchange their ions with sodium carbonate to produce calcium carbonate as a precipitate and sodium chloride in aqueous form.
Hence, the products form in the given reaction are, calcium carbonate,
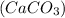 and sodium chloride,
and sodium chloride,