Reactant Favored - If there are more reactants than products then the reaction is reactants favored.
Product Favored - If there are more products than reactants after the reaction is completed then it is product favored.
Answer 1) Reactant favored
Explanation : 2
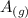 +3
+3
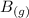 ----> 4
----> 4
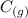 ΔH = -95 KJ
ΔH = -95 KJ
It is easily observed that the reactant has 5 moles in total whereas the product has only 4 moles. There fore, the number of reactant is more than the products. Hence, it is reactant favored reaction.
Answer 2) Reactant favored
Explanation : 2
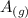 + 2
+ 2
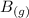 ---> 3
---> 3
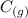 ΔH = 254 KJ.
ΔH = 254 KJ.
The total number of moles in reactant side are 2+2 =4 and in products side it has 3 moles only. This shows that products has less moles than reactants, and hence it is reactant favored reaction.
Answer 3) Product favoured
Explanation : 2
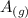 +
+
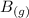 ---> 4
---> 4
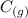 Δ H = 322 KJ
Δ H = 322 KJ
The total number of moles in reactant are 3=2+1=3 and the number of moles in products is 4. Which shoes that the number of moles in products is more than the reactants. This shows that it is product favored.
Answer 4) Product favored
Explanation : 2
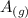 +
+
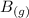 ----> 3
----> 3
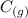 ΔH = -193 KJ
ΔH = -193 KJ
This is a product favored reaction as there are equal number of moles which are formed from reactants to products which is 3. But in this case, we can consider the ΔH is negative which is -193 KJ which shows that the reaction is product favored. And it shows that it is spontaneous in nature.