Answer: 0.942 ml
Step-by-step explanation:
To calculate volume of a substance, we use the equation:
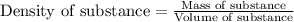
We are given:
Mass of gold= 18.2 g
Density of gold =
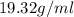
Volume of gold = volume of water displaced = ?
Putting values in above equation, we get:
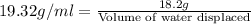
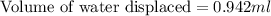
Hence, the volume of water, in milliliters, that would be displaced by a piece of gold weighing 18.2 g is 0.942.