Let's write the balanced chemical equation:
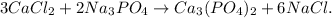
You can see that in the reactants and in the products we have the same number of atoms for each element. This is due to the matter of conservation law.
The total number of calcium atoms that are reacting and produced in the reaction is 3.