To solve this question we have to use the ideal gas law:
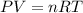
Where P is the pressure, V is the volume, n the number of moles, R the constant of ideal gases and T the temperature.
Solve the equation for T and replace for the given values. Remember that R has a value of 0.082atmL/molK:
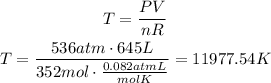
According to this, the temperature of the gas is 11977.54K.