Answer:
0.248 moles of CaO are produced.
Step-by-step explanation:
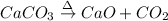
Moles of calcium carbonate = 0.248 moles
According to reaction, 1 mole of calcium carbonate gives 1 mole of calcium oxide and 1 mole of carbon dioxide.
Then 0.248 moles of calcium carbonate will give:
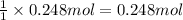 of calcium oxide
of calcium oxide
0.248 moles of CaO are produced.