Answer: The correct option would be A.
Step-by-step explanation: The main group elements which make more bonds than that was predicted from the octet rule are supposed to have expanded octet.
These elements tend to have more than 8 valence electrons after bonding and this can be achieved when we have empty d-orbitals.
When we have empty p-orbitals, total number of valence electrons than can be occupied will be 8.
Electronic configuration when valence shell's empty p-orbitals are fully filled =
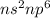
which means that a total of 8 electrons can be occupied which does not satisfy expanded octet rule.
Example of molecule showing expanded octet rule is given in the image. Here, after bonding Phosphorous has 10 electrons which is occupied in empty d-orbitals.