Answer:
The mass of the piece of aluminum is 645 g.
Step-by-step explanation:
Density is given by the relationship between mass and volume:
ρ =
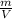
If we take de volume V and pass to multiply the density ρ, we have:
m = ρ * V (Equation 1)
with this formula we can find the mass.
We have that ρ = 2.70
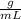 and V = 239mL
and V = 239mL
So, replacing these values in Equation 1, we can find the value for the mass:
m = 2.70
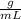 * 239mL
* 239mL
m = 645 g