Answer:
Number of moles of CO₂ formed = 0.375 moles
number of moles of water formed = 0.75 moles
Step-by-step explanation:
Acetylene reacts with oxygen according to the following chemical reaction:

The molar ratios are :
C₂H₂ = 2
O₂ = 5
CO₂ = 4
H₂O = 2
The basis is to find a limiting reagent which is given by the lowest number of moles.
The molar mass of acetylene is = 26.04
number of moles of acetylene =
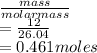
Number of moles of O₂ =
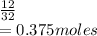
Number of moles of CO₂ formed = 0.375 moles
number of moles of water formed (2 x 0.375) = 0.75 moles