Answer:
Reaction c. has the largest value of
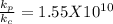 at 300 K
at 300 K
Step-by-step explanation:
Here is the complete question
Converting between Kc and Kp For which of the following reactions is the ratio Kp/Kc largest at 300 K?
a. N2(g) + O2(g) ⇌ 2 NO(g)
b. C(s) +2H2(g) ⇌ CH4(g)
c. Ni(CO)4(g) ⇌ Ni(s) + 4CO(g)
d. CaCO3(s) ⇌ CaO(s) + CO2(g)
Solution
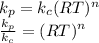
where R = molar gas constant = 8.314 J/mol-K, T = temperature = 300 K and n = number of moles of products - number of moles of reactants
For reaction a. number of moles of gaseous products = 2, number of moles of gaseous reactants = 2. So n = 2 - 2 = 0
So,
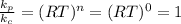
For reaction b. number of moles of gaseous products = 1, number of moles of gaseous reactants = 2. So n = 1 - 2 = -1 (we do not include the solid)
So,
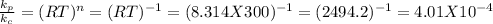
For reaction c. number of moles of gaseous products = 5, number of moles of gaseous reactants = 1. So n = 4 - 1 = 3 (we do not include the solid)
So,
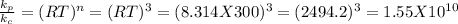
For reaction d. number of moles of gaseous products = 2, number of moles of gaseous reactants = 0. So n = 2 - 0 = 2 (we do not include the solid)
So,
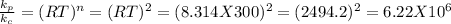
Since
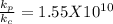 for reaction c which is the largest value. So, reaction c. has the largest value of
for reaction c which is the largest value. So, reaction c. has the largest value of
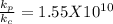 at 300 K
at 300 K