Answer:
According to boyle’s law, the volume of a gas is inversely proportional to its pressure if the Temperature = constant
Step-by-step explanation:
As per ideal gas equation we know that
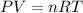
here we know that
n = number of moles
R = gas constant
T = temperature of gas
V = volume of gas
P = pressure of gas
so here we know that if the system is closed and temperature is constant then in that case
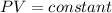
so pressure is inversely depends on the volume of the gas
so correct answer will be
According to boyle’s law, the volume of a gas is inversely proportional to its pressure if the Temperature = constant