Answer:
The given statement is false.
Step-by-step explanation:
Given that aluminum boils at 2,467°C
Boiling point of aluminum in kelvins is 2,194 K
Temperature in degree Celsius can be converted into Kelvins by relation:
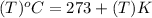
So, boiling point of aluminium in Kelvins will be:
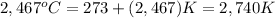
2,740 K ≠ 2,194 K
But the given value in Kelvins is not coming equal to our calculated value, Hence, the given statement is false.