Answer: The final energy is 1,700 J
Explanation: According to first law of thermodynamics:
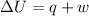
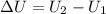 =Final energy-initial energy=Change in internal energy
=Final energy-initial energy=Change in internal energy
q = heat absorbed or released
w = work done by or on the system
w = work done by the system=
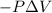 {Work done by the system is negative as the final volume is greater than initial volume}
{Work done by the system is negative as the final volume is greater than initial volume}
q = +500J {Heat absorbed by the system is positive}
w = work done by the system = -300J

