Answer:
The mass of calcium in the given number of atoms is 797.2 g.
Step-by-step explanation:
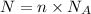
N = Number of atoms/molecules
n = Moles of compound
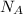 = Avogadro number =
= Avogadro number =
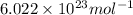
We have:
Number calcium atoms =
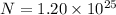
n = moles of calcium = ?
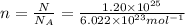
n = 19.93 mol
Atomic mass of calcium = 40 g/mol
Mass of 19.93 moles of calcium = 40 g/mol × 19.93 mol=797.2 g
The mass of calcium in the given number of atoms is 797.2 g.