Answer:
There are 3.594 moles of sulfur dioxide.
Step-by-step explanation:
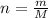
n = moles of compound
m = mass of the compound
M = Molar mass of the compound
Mass of sulfur dioxide= m = 0.23 kg = 230 g (1 kg = 1000 g)\
Molar mass of sulfur dioxde = M = 64 g/mol
Moles of sulfur dioxide = n
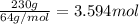
There are 3.594 moles of sulfur dioxide.