Answer:
hydronium ion concentration will decrease by factor of 100
Step-by-step explanation:
As we know that form to find pH of the solution is given as
![pH = -Log[H^+]](https://img.qammunity.org/2018/formulas/physics/high-school/oiyhclo0xnamq0yc2q49enqwpfryl0bagj.png)
so here we know that pH of the solution is decreased by 2
so we have

now using above formula
![2 = -Log[H_1^+] + Log[H_2^+]](https://img.qammunity.org/2018/formulas/physics/high-school/r6gok86sbfa3p5i64tm5qzj5cbwavsv8ql.png)
so we will have
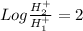
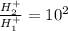
so hydronium ion concentration will decrease by factor of 100