Hello!
If a sample contains 21.2 g N, how many moles of N does it contain?
0.66 mol
1.51 mol
14.01 mol
297.01 mol
We have the following data:
m (mass) = 21.2 g
MM (Molar mass of Nitrogen) = 14 g / mol
n (number of moles) = ?
Formula:
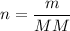
Solving:
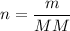
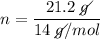
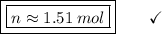
Answer:
1.51 mol
_______________________________
I Hope this helps, greetings ... Dexteright02! =)