Step-by-step explanation:
Percentage of an element in a compound:
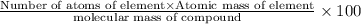
Molecular mass of
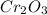 = 152 g/mol
= 152 g/mol
Percentage of chromium:
Number of chromium atoms = 2
Atomic mass of chromium = 52 g/mol
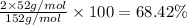
Percentage of oxygen:
Number of oxygen atoms = 3
Atomic mas of oxygen = 16 g/mol
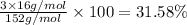
The percent composition of Cr in
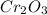 is 68.42%.
is 68.42%.
The percent composition of O in
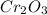 is 31.58%.
is 31.58%.