Answer: The correct statements are volume of a substance and density of a substance.
Step-by-step explanation:
Degree of intermolecular bonds is defined as the forces by which two particles are bonded in a substance. If the intermolecular forces are strong, it means that it will occupy less space or volume and hence, the density of that substance will be more.
If the intermolecular forces are weak, it means that it will occupy less space and density of the substance will be more.
Relationship between volume and density of a substance is given by the relation:
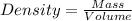
Density and volume has inverse relationship. If volume increases, density will decrease and vice-versa.
Hence, the correct statements are volume of a substance and density of a substance.