Answer:
Atomic radius,

Step-by-step explanation:
It is given that,
Density of nickel, d = 8.9 g/cm³
The density of a unit cell is given by :
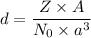
Where
Z = no of atoms per unit cell
A = molar mass of an element in g/mol
N₀ = Avogadro's number
a = edge length
The edge length of FCC crystal is,
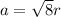 .............(1)
.............(1)
r = atomic radius
Nickel has a FCC structure and for FCC, Z = 4
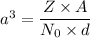
For nickel A = 58.69 g/mol
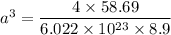
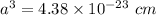

Atomic radius is,
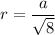
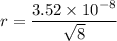

Hence, this is the required solution.