Answer: Boron -10 : 20%
Boron -11 : 80%
Step-by-step explanation:
Mass of isotope boron -10= 10
% abundance of isotope 1 = x% =
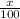
Mass of isotope boron-11 = 11
% abundance of isotope 2 = (100-x)% =
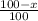
Formula used for average atomic mass of an element :
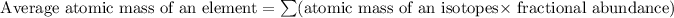
![10.8=\sum[(10)* (x)/(100))+(11)* (100-x)/(100)]]](https://img.qammunity.org/2018/formulas/chemistry/high-school/ldmhrmd01p8dyp877advlpfq82snam0km0.png)
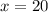
Therefore, percent of boron -10 is 20% and isotope boron-11 is (100-20)= 80%.