Answer : The maximum concentration of silver ion is

Solution : Given,
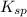 for AgBr =
for AgBr =
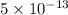
Concentration of NaBr solution = 0.1 m
The equilibrium reaction for NaBr solution is,

The concentration of NaBr solution is 0.1 m that means,
![[Na^+]=[Br^-]=0.1m](https://img.qammunity.org/2018/formulas/physics/high-school/z13shmxanownwomkhnwofogcub2qiyiuy8.png)
The equilibrium reaction for AgBr is,
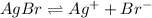
At equilibrium s s
The expression for solubility product constant for AgBr is,
![K_(sp)=[Ag^+][Br^-]](https://img.qammunity.org/2018/formulas/physics/high-school/xdo6kxn84heedkyarooug2vmkbkzmz0lzd.png)
The concentration of
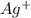 = s
= s
The concentration of
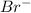 = 0.1 + s
= 0.1 + s
Now put all the given values in
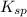 expression, we get
expression, we get
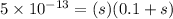
By rearranging the terms, we get the value of 's'
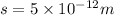
Therefore, the maximum concentration of silver ion is
 .
.