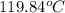Answer : The final temperature of water is,
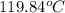
Solution :
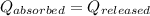
As we know that,
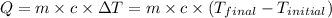
![m_1* c_g* (T_(final)-T_2)=-[m_2* c_w* (T_(final)-T_1)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/c084og74ziq82gdzv7xbfaxxw64z3j060x.png) .................(1)
.................(1)
where,
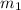 = mass of glass = 32.50 g
= mass of glass = 32.50 g
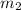 = mass of water = 57 g
= mass of water = 57 g
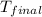 = final temperature of water and glass =
= final temperature of water and glass =
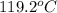
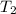 = initial temperature of water = ?
= initial temperature of water = ?
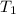 = initial temperature glass =
= initial temperature glass =
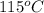
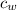 = specific heat of water =
= specific heat of water =
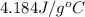
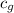 = specific heat of glass =
= specific heat of glass =
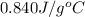
Now put all the given values in equation (1), we get
![m_1* c_g* (T_(final)-T_2)=-[m_2* c_w* (T_(final)-T_1)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/c084og74ziq82gdzv7xbfaxxw64z3j060x.png)
![(32.50g)* (0.840J/g^oC)* (119.2^oC-115^oC)=-[(57g)* (4.184J/g^oC)* (119.2^oC-T_1)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/yro6f76sbv368zuhu52o1ibx1nxobb4q19.png)
Therefore, the final temperature of water is,