Answer:The correct answer is option d.
Step-by-step explanation:
Molarity of the solution = 2.5 M =2.5 mol/L
Number of moles of glucose = n
Molarity is defined as ratio of moles of solute to the volume of the solutions in liters.
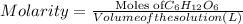
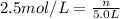
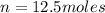
The closest option to our answer is option d.
Hence,correct answer is option d.