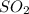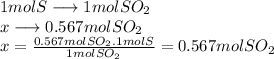Answer:
Step-by-step explanation:
We have the reaction

we balance the equation

In this case the equation is already balanced
We use stoichiometric relations to solve it.
we know that 1 mol of sulfur produces 1 mol of
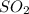 (we know it by the coefficients of balancing of the equation, which in this are equal to 1)
(we know it by the coefficients of balancing of the equation, which in this are equal to 1)
Now how many moles of sulfur are needed to produce 0.567 moles of