Answer:
+1
Step-by-step explanation:
Sodium is the element of first group and third period. The electronic configuration of sodium is - 2, 8, 1 or
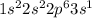
There is one valence electron of sodium.
If sodium loses one electron, then it will attain the noble gas configuration of neon and become stable. By losing one electron, it will become positively charged.
Thus, the charge on sodium element will be +1.