Answer:
11.347 g/mL is the density of the metal pellets.
Step-by-step explanation:
The total mass of the flask, metal, and methanol = M = 142.412 g
Mass of flask = 25.00 mL
Mass of flask and metal pellets = 130.278 g
mass of flask + mass of pellets = 130.270 g
M = mass of flask + mass of pellets + mass of methanol
142.412 g = 130.270 g + mass of methanol
Mass of methanol = 12.142 g
Volume of methanol = v
Density of the methanol = 0.7918 g/mL
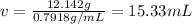
Volume of the flask = V = 25 mL
Volume of the pellets = V'
V = V' + v
V'= V - v = 25 mL - 15.33 mL = 9.67 mL
Density of the metal pellets = d
Mass of the metal pellets = m
Mass of flask and metal pellets = 130.278 g
20.55 g + m = 130.278 g
m = 109.728 g
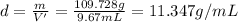
11.347 g/mL is the density of the metal pellets.