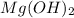Answer : The X and Y are,
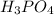 and
and
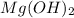
Explanation :
Acid-base reaction : It is a neutralization reaction in which an acid react with a base to give salt and water as a product.
When phosphoric acid react with the magnesium hydroxide base to give magnesium phosphate and water as a product.
The balanced acid-base reaction will be,
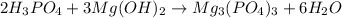
X =
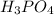 = phosphoric acid = Acid
= phosphoric acid = Acid
Y =
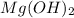 = magnesium hydroxide = Base
= magnesium hydroxide = Base
Hence, the X and Y are,
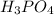 and
and