Answer:
Two alcohol functional groups
Step-by-step explanation:
Hello,
Condensation polymerizations are based on the releasing of water after the undergoing chemical reactions. In this manner, an illustrative example is shown below:
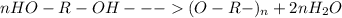
This is caused by high reactivity of the hydroxide ions when they are together allowing the polymerization to take place.
Best regards.